Klassifizierung der zellbasierten
Therapie-Technologie
Es wird deutlich, dass sich die Landschaft des Entwicklungsstandes und des Einsatzes von Zelltherapien
in den kommenden Jahren aufgrund der sehr positiven Wirksamkeitsdaten im
Bereich der Immunzelltherapie erheblich verändern wird.
Somatische Zelltechnologien
Technologien zur Immortalisierung von Zellen
Technologien zur Zellplastizität
Treffen Sie unser Team

Melanie Strauss

René Schweizer

Claudia Luft
Neue Wege für toleranzinduzierende Zelltherapien

Tolerogene Therapie
Die tolerogene Therapie zielt darauf ab, Immuntoleranz zu induzieren, wenn eine pathologische oder unerwünschte Aktivierung der normalen Immunantwort vorliegt.
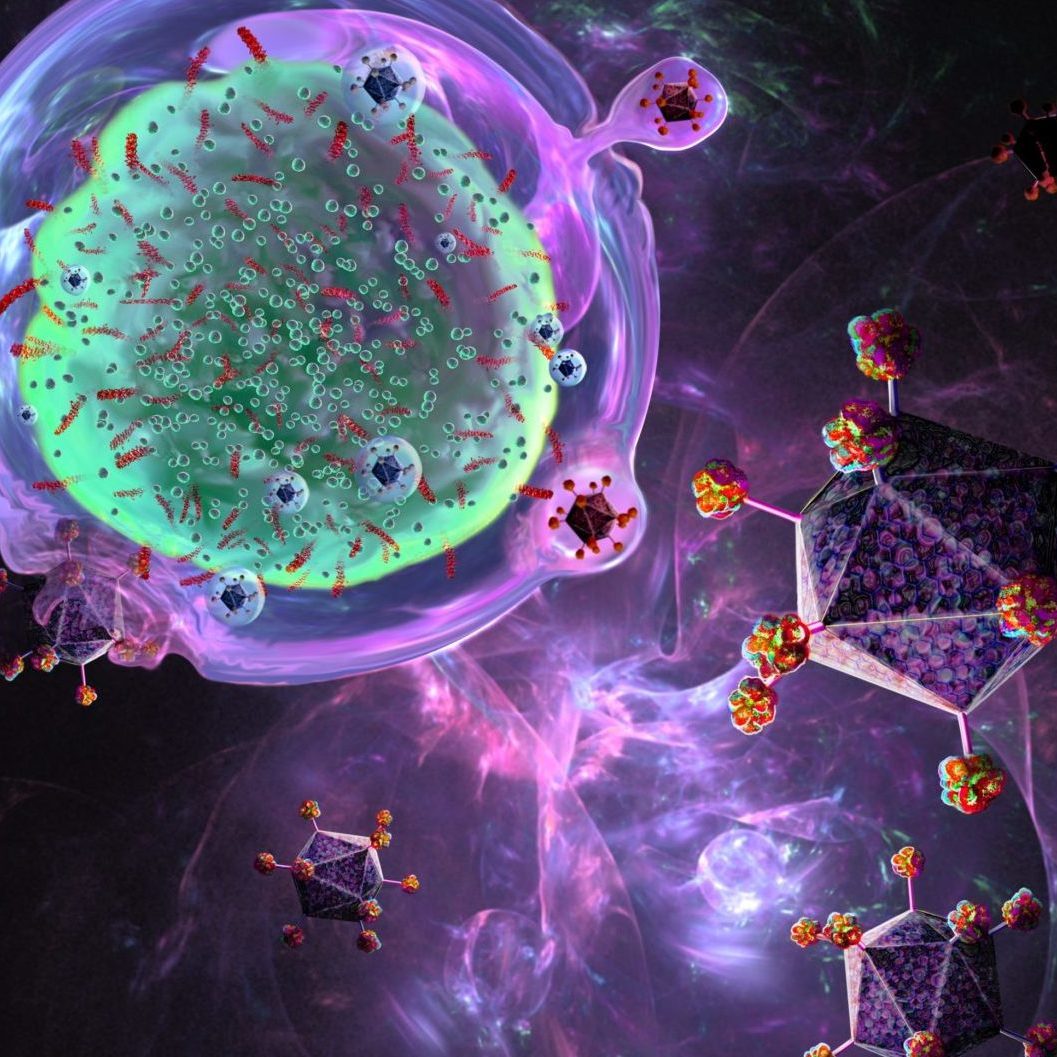
CAR-T-Zell-Therapie
Chimäre Antigenrezeptor-T-Zellen sind T-Zellen, die gentechnisch so verändert wurden, dass sie einen künstlichen T-Zell-Rezeptor für den Einsatz in der Immuntherapie produzieren.
Gentechnisch veränderte TCR-Therapie
Bei der TCR-Therapie werden T-Zellen genetisch so verändert, dass sie einen neuen Rezeptor produzieren, der sie in die Lage versetzt, sich an bestimmte Zielproteine auf Tumorzellen zu heften.
Neueste Nachrichten und Artikel

Entwicklung zellbasierter Therapien
Bevor neue Behandlungen beim Patienten ankommen, müssen sie zunächst an den Bausteinen des Körpers getestet werden.
Wir alle bestehen aus einer Ansammlung vielfältiger und spezialisierter Zellen, die zusammenarbeiten, um die Prozesse des Lebens auszuführen. Durch die Untersuchung dieser mikroskopischen Bausteine des Körpers können wir viel über die menschliche Gesundheit und Krankheit lernen. Durch die Schaffung von Zellmodellen mit Merkmalen, die bei bestimmten Krankheiten vorkommen, können Wissenschaftler die Funktionsstörungen, die Krankheiten zugrunde liegen, aufklären und Therapien zu ihrer Behebung testen.
Die Wissenschaftler des IRP haben große Fortschritte bei der Erforschung gesunder und defekter Zellen gemacht. Unsere Forscher haben zelluläre Prozesse aufgeklärt, z. B. wie sich Zellen an den richtigen Ort im Körper bewegen, wie Zellen die Aktivität ihrer Gene steuern und wie umgeordnete DNA-Stücke zu Krebs führen können. Diese Art von zellbasierten Studien haben zu Behandlungen für Krankheiten von Krebs über Arthritis bis hin zu seltenen primären Immunschwächekrankheiten (PIDDs) geführt. Insbesondere gehörten die IRP-Forscher zu den ersten Wissenschaftlern, die sich mit der Idee beschäftigten, das Immunsystem für die Krebsbekämpfung zu trainieren, was zu dem innovativen Gebiet der Immuntherapie und der Entwicklung erfolgreicher Behandlungen für zahlreiche zuvor unheilbare Tumore führte. Das IRP hat auch einen bedeutenden Beitrag zur Gentherapie geleistet, indem es 1990 die allererste Gentherapie getestet hat.
Fortschritte bei zellbasierten Therapien, insbesondere bei der Verwendung induzierter pluripotenter Stammzellen (iPSC), die in jeden Zelltyp umgewandelt werden können, könnten bald die Art und Weise revolutionieren, wie Kliniker HIV, Krebs, neurologische Erkrankungen wie amyotrophe Lateralsklerose (ALS) und Autoimmunerkrankungen wie Typ-1-Diabetes und Morbus Crohn behandeln. Zur Unterstützung der IRP-Forscher, die in diesem Bereich arbeiten, stellt das NIH-Programm für regenerative Medizin (RMP) Ressourcen zur Verfügung, um die Umsetzung zellbasierter Entdeckungen in wirksame medizinische Maßnahmen zu beschleunigen und die zahlreichen klinischen Versuche dieser Therapien zu unterstützen, die am NIH Clinical Center durchgeführt werden. Das RMP hat außerdem Stammzelllinien für den Einsatz in der Forschung entwickelt, Standardverfahren für die Herstellung und Verwendung der Zellen geschaffen, Schulungskurse konzipiert und Partnerschaften für die Zelllagerung aufgebaut. Darüber hinaus hat das RMP 2015 das Stem Cell Translation Laboratory (STCL) ins Leben gerufen, ein hochmodernes Forschungszentrum, das Wissenschaftlern dabei helfen soll, die wissenschaftlichen und technologischen Hürden der iPSC-Forschung zu überwinden.
Mit diesen Programmen, die ihre Arbeit unterstützen, machen die IRP-Forscher ständig neue Entdeckungen darüber, wie unsere Zellen funktionieren, und erzielen bahnbrechende Erfolge bei der Behandlung zahlreicher Krankheiten. Unsere Forscher werden weiterhin zellbasierte Therapien vorantreiben, indem sie:
Erforschung der grundlegenden Prozesse gesunder und kranker Zellen, von der Zellteilung und dem Zelltod bis zur Energiegewinnung und dem Transport biologischer Materialien.
Reprogrammierung von Immunzellen, um sie dazu zu bringen, Krebszellen anzugreifen
Einführung von Qualitätskontrollverfahren für die Herstellung und Verwendung von Stammzelllinien.
Entwicklung neuer, hochmoderner Moleküle, die die derzeitigen Forschungsmaterialien ergänzen oder ersetzen.
Action to Focus and Accelerate Cell-based Tolerance-inducing Therapies
A FACTT is pushing the limits, inviting you to come along and join us !
WHY A FACTT ?
The number of patients with autoimmune diseases and recipients of organ or stem-cell transplants is increasing worldwide. Currently, these patients require lifelong administration of immunosuppressive drugs. Often, these drugs are ineffective, expensive and show severe side-effects. More effective and safer therapies aimed at modifying unwanted immune responses permanently or for prolonged periods are needed.
Accumulating knowledge on mechanisms of immune tolerance has led to development of specific Cell-based Tolerance-inducing Therapies (CTT) with the specific objectives to restrain unwanted immune reactions long-term. For patients, personalised CTT will represent a breakthrough for healthcare and quality of life.
Clinical CTT studies are underway or starting in single European institutes. The used CTT products are diverse and the efficacy of therapy is monitored by different parameters. This creates the risk of redundancy in trials and suboptimal performance indicators for comparison of trial outcomes.
The main objective of A FACTT is to initiate a network that will coordinate European CTT efforts to minimise overlap and maximise comparison of the diverse approaches through establishment of consensus monitoring parameters. The A FACTT-Action will thus focus and accelerate the CTT field and ensure that the field will progress in an efficient, safe and cost-effective way.
The A FACTT Project
Do you like to quickly learn more about the A FACTT project ? Please click here and find all information about the project, its objectives, its background and the benefits, as well as your starting point to the scientific work being carried out.Read More & Discover A FACTT
How to join A FACTT
Are you interested in the A FACTT project and would you like to join us and become part of Europe’s biggest network on Cell-based Tolerance-inducing Therapies ? Please apply here !Read More & Discover A FACTT
Contact A FACTT

